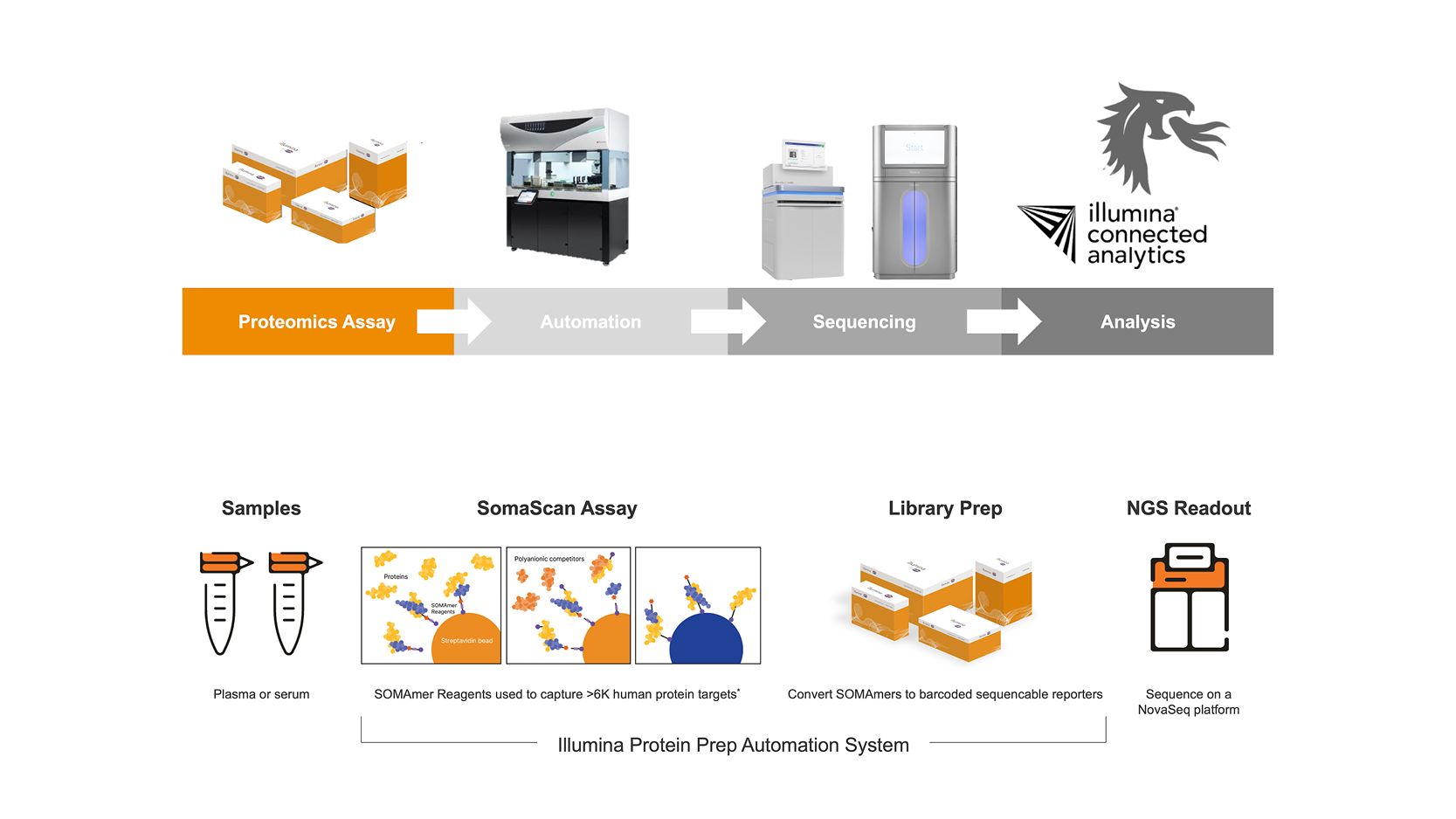
NGS-ENABLED CONCURRENT MEASUREMENT OF >6K PLASMA PROTEINS
The Illumina Protein Prep Workflow is an end-to-end automated solution that seamlessly integrates the unparalleled sensitivity and quantitative precision of the SomaScan assay with Illumina’s Next Generation Sequencing (NGS) technology, offering unmatched throughput, dynamic range, and precision in protein analysis from human serum and plasma samples.
This innovative proteomics assay uses SOMAmer® (slow off-rate modified aptamer) Reagents for protein capture to achieve high specificity for target proteins compared to antibody-based approaches. Combining this advanced proteomics assay with NGS-based readout and the bioinformatics power of Illumina data analysis software provides a streamlined sample-to-results solution for evaluating more than 6000 unique human proteins in a single plasma or serum sample.

Comprehensive, high throughput, proteomics analysis using NGS technology is conducted by highly trained staff to provide over 6500 unique protein measurements. Using only 55 µL of plasma or serum per sample, our service includes complete sample preparation with proprietary 6K SOMASCAN Assay for NGS, data acquisition on Illumina NGS platform, data analysis from base Quality Control (QC) metrics to full publication-ready reports, including custom analyses tailored to your specific needs.
Our assay comes with three tiered options for data reporting, each available for purchase. Each reporting tier offers varying degrees of detail and analysis to suit different customer needs.
Level 1: Basic Analysis
Level 2: Intermediate Analysis
All of the above plus
Level 3: Custom Analysis
The academic rate is $630 per sample by increments of 170 samples (2 ProteinPrep plates, 85 samples per plate).
Please contact us [email protected] for commercial pricing and volume discounts.
Turnaround Time (TAT)
Typical estimated TAT from sample reception to report delivery (Level 1: Basic Analysis) is three weeks. Please note that turnaround times are estimated and not guaranteed. Specimen quality, record completeness, instrument availability and maintenance schedules are just some of the factors that can affect TAT.
MEET THE TEAM